1
For the latest news, follow us on LinkedIn!
Data freeze 12 results and summary statistics now available!
Results can be browsed online using the FinnGen web browser and the summary statistics downloaded
This data freeze consists of >500,000 individuals, more than 21.3 M variants and 2,500 health endpoints. To get more information about how to access the results and summary statistics, please follow this link:

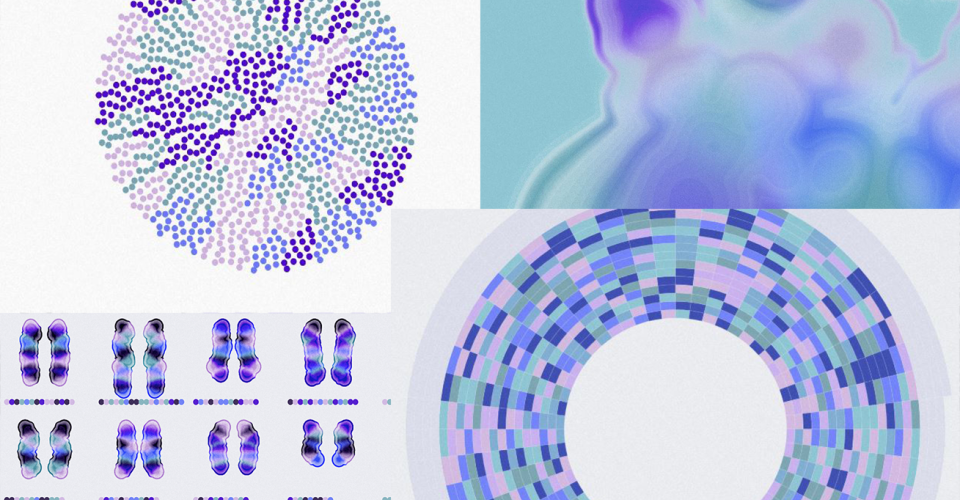
New accessible and engaging FinnGen communication resource available!
Scroll through the website to learn about the FinnGen project, its goals, processes and its impact in Finland and around the world.
Can you imagine what genetic diversity looks like in large populations or visualise where genes are located on chromosomes in relation to each other? We teamed up with Aalto University to produce a resource that uses data visualisation to tell the FinnGen story to a wider audience.
News
FinnGen brings together the nation-wide network of Finnish biobanks.
Every Finn can be a part of the FinnGen study by giving a biobank consent.
Samples available
629 000
Samples needed by 2023: 520 000
Current data freeze
520 210
combined genotype and health registry data


